As noted in a previous chapter, the light our eyes see is but a small part of a broad spectrum of electromagnetic radiation. On the immediate high energy side of the visible spectrum lies the ultraviolet, and on the low energy side is the infrared. The portion of the infrared region most useful for analysis of organic compounds is not immediately adjacent to the visible spectrum, but is that having a wavelength range from 2,500 to 16,000 nm, with a corresponding frequency range from 1.9*1013 to 1.2*1014 Hz.
| |||||
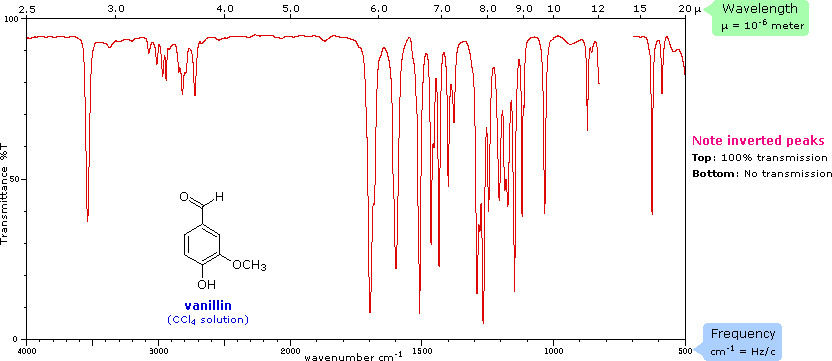
The frequency scale at the bottom of the chart is given in units of reciprocal centimeters (cm-1) rather than Hz, because the numbers are more manageable. The reciprocal centimeter is the number of wave cycles in one centimeter; whereas, frequency in cycles per second or Hz is equal to the number of wave cycles in 3*1010 cm (the distance covered by light in one second). Wavelength units are in micrometers, microns (μ), instead of nanometers for the same reason. Most infrared spectra are displayed on a linear frequency scale, as shown here, but in some older texts a linear wavelength scale is used. A calculator for interconverting these frequency and wavelength values is provided on the right. Simply enter the value to be converted in the appropriate box, press "Calculate" and the equivalent number will appear in the empty box.
Infrared spectra may be obtained from samples in all phases (liquid, solid and gaseous). Liquids are usually examined as a thin film sandwiched between two polished salt plates (note that glass absorbs infrared radiation, whereas NaCl is transparent). If solvents are used to dissolve solids, care must be taken to avoid obscuring important spectral regions by solvent absorption. Perchlorinated solvents such as carbon tetrachloride, chloroform and tetrachloroethene are commonly used. Alternatively, solids may either be incorporated in a thin KBr disk, prepared under high pressure, or mixed with a little non-volatile liquid and ground to a paste (or mull) that is smeared between salt plates.
2. Vibrational Spectroscopy
A molecule composed of n-atoms has 3n degrees of freedom, six of which are translations and rotations of the molecule itself. This leaves 3n-6 degrees of vibrational freedom (3n-5 if the molecule is linear). Vibrational modes are often given descriptive names, such as stretching, bending, scissoring, rocking and twisting. The four-atom molecule of formaldehyde, the gas phase spectrum of which is shown below, provides an example of these terms. If a ball & stick model of formaldehyde is not displayed to the right of the spectrum, press the view ball&stick model button on the right. We expect six fundamental vibrations (12 minus 6), and these have been assigned to the spectrum absorptions. To see the formaldehyde molecule display a vibration, click one of the buttons under the spectrum, or click on one of the absorption peaks in the spectrum.
Gas Phase Infrared Spectrum of Formaldehyde, H2C=O | ||
|---|---|---|
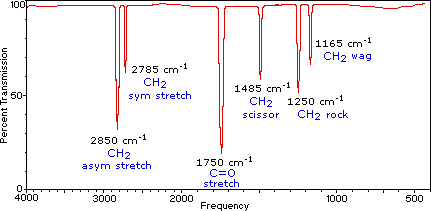 | ||
View CH2 Symmetric Stretch View C=O Stretch View CH2 Scissoring View CH2 Rocking View CH2 Wagging | Ball&Stick Model Spacefill Model Stick Model Motion Off | |
The exact frequency at which a given vibration occurs is determined by the strengths of the bonds involved and the mass of the component atoms. For a more detailed discussion of these factors Click Here. In practice, infrared spectra do not normally display separate absorption signals for each of the 3n-6 fundamental vibrational modes of a molecule. The number of observed absorptions may be increased by additive and subtractive interactions leading to combination tones and overtones of the fundamental vibrations, in much the same way that sound vibrations from a musical instrument interact. Furthermore, the number of observed absorptions may be decreased by molecular symmetry, spectrometer limitations, and spectroscopic selection rules. One selection rule that influences the intensity of infrared absorptions, is that a change in dipole moment should occur for a vibration to absorb infrared energy. Absorption bands associated with C=O bond stretching are usually very strong because a large change in the dipole takes place in that mode.
Some General Trends:
ii) Bonds to hydrogen have higher stretching frequencies than those to heavier atoms.
iii) Triple bonds have higher stretching frequencies than corresponding double bonds, which in turn have higher frequencies than single bonds.
(Except for bonds to hydrogen).
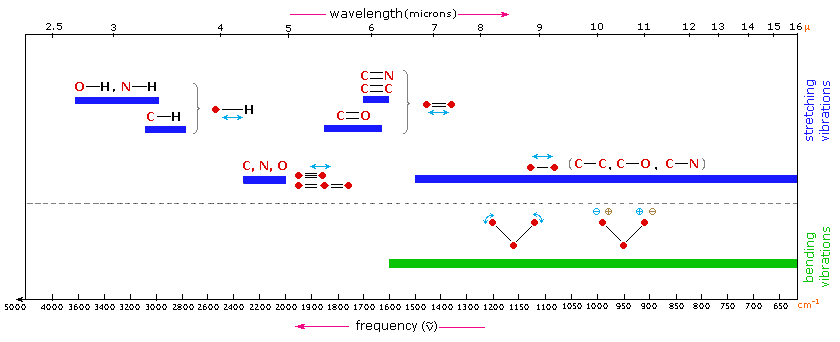
Detailed information about the infrared absorptions observed for various bonded atoms and groups is usually presented in tabular form. The following table provides a collection of such data for the most common functional groups. Following the color scheme of the chart, stretching absorptions are listed in the blue-shaded section and bending absorptions in the green shaded part. More detailed descriptions for certain groups (e.g. alkenes, arenes, alcohols, amines & carbonyl compounds) may be viewed by clicking on the functional class name. Since most organic compounds have C-H bonds, a useful rule is that absorption in the 2850 to 3000 cm-1 is due to sp3 C-H stretching; whereas, absorption above 3000 cm-1 is from sp2 C-H stretching or sp C-H stretching if it is near 3300 cm-1.
| Stretching Vibrations | Bending Vibrations | |||||
|---|---|---|---|---|---|---|
| Functional Class | Range (cm-1) | Intensity | Assignment | Range (cm-1) | Intensity | Assignment |
| Alkanes | 2850-3000 | str | CH3, CH2 & CH 2 or 3 bands | 1350-1470 1370-1390 720-725 | med med wk | CH2 & CH3 deformation CH3 deformation CH2 rocking |
| Alkenes | 3020-3100 1630-1680 1900-2000 | med var str | =C-H & =CH2 (usually sharp) C=C (symmetry reduces intensity) C=C asymmetric stretch | 880-995 780-850 675-730 | str med med | =C-H & =CH2 (out-of-plane bending) cis-RCH=CHR |
| Alkynes | 3300 2100-2250 | str var | C-H (usually sharp) C≡C (symmetry reduces intensity) | 600-700 | str | C-H deformation |
| Arenes | 3030 1600 & 1500 | var med-wk | C-H (may be several bands) C=C (in ring) (2 bands) (3 if conjugated) | 690-900 | str-med | C-H bending & ring puckering |
| Alcohols & Phenols | 3580-3650 3200-3550 970-1250 | var str str | O-H (free), usually sharp O-H (H-bonded), usually broad C-O | 1330-1430 650-770 | med var-wk | O-H bending (in-plane) O-H bend (out-of-plane) |
| Amines | 3400-3500 (dil. soln.) 3300-3400 (dil. soln.) 1000-1250 | wk wk med | N-H (1°-amines), 2 bands N-H (2°-amines) C-N | 1550-1650 660-900 | med-str var | NH2 scissoring (1°-amines) NH2 & N-H wagging (shifts on H-bonding) |
| Aldehydes & Ketones | 2690-2840(2 bands) 1720-1740 1710-1720 | med str str str str str str | C-H (aldehyde C-H) C=O (saturated aldehyde) C=O (saturated ketone) aryl ketone α, β-unsaturation cyclopentanone cyclobutanone | 1350-1360 1400-1450 1100 | str str med | α-CH3 bending α-CH2 bending C-C-C bending |
| Carboxylic Acids & Derivatives | 2500-3300 (acids) overlap C-H 1705-1720 (acids) 1210-1320 (acids) | str str med-str str str str str str str | O-H (very broad) C=O (H-bonded) O-C (sometimes 2-peaks) C=O C=O (2-bands) O-C C=O O-C (2-bands) C=O (amide I band) | 1395-1440 1590-1650 1500-1560 | med med med | C-O-H bending N-H (1°-amide) II band N-H (2°-amide) II band |
| Nitriles Isocyanates,Isothiocyanates, Diimides, Azides & Ketenes | 2240-2260 2100-2270 | med med | C≡N (sharp) -N=C=O, -N=C=S -N=C=N-, -N3, C=C=O | |||
To illustrate the usefulness of infrared absorption spectra, examples for five C4H8O isomers are presented below their corresponding structural formulas. The five spectra may be examined in turn by clicking the "Toggle Spectra" button. Try to associate each spectrum (A - E) with one of the isomers in the row above it. When you have made assignments check your answers by clicking on the structure or name of each isomer.
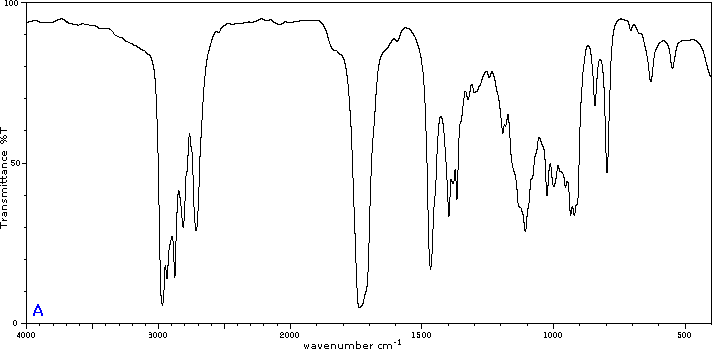
Infrared absorption data for some functional groups not listed in the preceding table are given below. Most of the absorptions cited are associated with stretching vibrations. Standard abbreviations (str = strong, wk = weak, brd = broad & shp = sharp) are used to describe the absorption bands.
| Functional Class | Characteristic Absorptions |
|---|---|
| Sulfur Functions | |
| S-H thiols | 2550-2600 cm-1 (wk & shp) |
| S-OR esters | 700-900 (str) |
| S-S disulfide | 500-540 (wk) |
| C=S thiocarbonyl | 1050-1200 (str) |
| S=O sulfoxide | 1030-1060 (str) 1325± 25 (as) & 1140± 20 (s) (both str) 1345 (str) 1365± 5 (as) & 1180± 10 (s) (both str) 1350-1450 (str) |
| Phosphorous Functions | |
| P-H phosphine | 2280-2440 cm-1 (med & shp) 950-1250 (wk) P-H bending |
| (O=)PO-H phosphonic acid | 2550-2700 (med) |
| P-OR esters | 900-1050 (str) |
| P=O phosphine oxide | 1100-1200 (str) 1230-1260 (str) 1100-1200 (str) 1200-1275 (str) |
| Silicon Functions | |
| Si-H silane | 2100-2360 cm-1 (str) |
| Si-OR | 1000-1110 (str & brd) |
| Si-CH3 | 1250± 10 (str & shp) |
| Oxidized Nitrogen Functions | |
| =NOH oxime | 3550-3600 cm-1 (str) 1665± 15 945± 15 |
| N-O amine oxide | 960± 20 1250± 50 |
| N=O nitroso | 1550± 50 (str) 1530± 20 (as) & 1350± 30 (s) |
0 comments:
Post a Comment